Antibiotic awareness: Spreading knowledge on antibacterial resistance
Food, from animal production, has undergone a dramatic change since antibiotics began being used for food preservation, on farms and aquaculture operations in the 1930s. As with other animal production operations, the poultry industry began to keep large numbers of animals in smaller spaces, making it easier for diseases to be transmitted. Agricultural antibiotics helped treat infections and adding them to feed had a positive response through increased feed conversion and faster growth. The success realized with antibiotics led to the widespread adoption in the early 1950s into several food production industries.

Antibiotics are used for therapeutic, prophylactic, sub-therapeutic and therapeutic applications. Therapeutic use is for treating an already existing infection. Prophylactic applications are for the prevention of imminent disease in healthy animals, with sub-therapeutic use mainly for growth promotion. Sub-therapeutic antibiotics are also referred to as antibiotic growth promoters (AGPs). Often, an existing bacterial infection is treated with the highest dosage being applied, while subtherapeutic applications use the lowest doses.
The first warnings regarding the potential for bacteria to develop resistance to antibiotics came as early as 1955 from Alexandra Fleming. Fleming was concerned that small doses could cause a build-up of resistant strains. Several reports confirming the existence of antibiotic-resistant strains of e-coli were reported in 1960. It was suspected that the resistant strains might have developed in animals and were passed on to the human population through ingestion. Antibiotic bans and restrictions were slow to be implemented, mainly due to fragmented concerns and a lack of meaningful international agreements.
In 1969, the Swann committee released a series of reforms restricting medically important antibiotics from being used as agricultural antibiotics.
This was a significant step towards antibiotic control by categorising antibiotics for human
health use or veterinary use. At the farm level, efforts to reduce the prophylactic use of antibiotics and for growth promotion have also been slow, owing to their success in lowering costs for treating infections and their benefits for improving feed conversion efficiencies. Also, reports on the risks of antibiotic-resistant microbes are always in the future, and people did not perceive the risks as urgent. This is still true today.
Responsible use of antibiotics
Some progress has been made towards regulating, restricting, and reducing antibiotic use in animal production. In 2006, the European Union, including the United Kingdom, banned the use of all antibiotic growth promoters in farm animals. In 2015, the World Health organisation endorsed a Global action plan to raise public awareness and optimise the use of antibiotics. An article in The Lancet Planetary Health found that interventions that restrict antibiotic use in food-producing animals reduced antibiotic-resistant bacteria in these animals by up to 39%. This research directly informed the development of WHO’s new guidelines. In 2005, Korea added over 45 antimicrobial feed additives to the list, requiring a veterinarian to give a script before use. Countries such as Japan have committed to reducing their antibiotic use by a third by 2020. Middle- and low-income countries such as Brazil, China, and Vietnam have committed to reducing, restricting, and banning some antibiotics following WHO’s guidelines.
The banning of AGPs will probably result in production losses for the livestock industry. However, it may refocus efforts towards preventing diseases through improved hygiene and strengthening biosecurity measures. The adoption of the One Health concept by WHO is another way to achieve better health across multiple sectors, with the single purpose of improving health in people, animals, plants, and their shared environment.
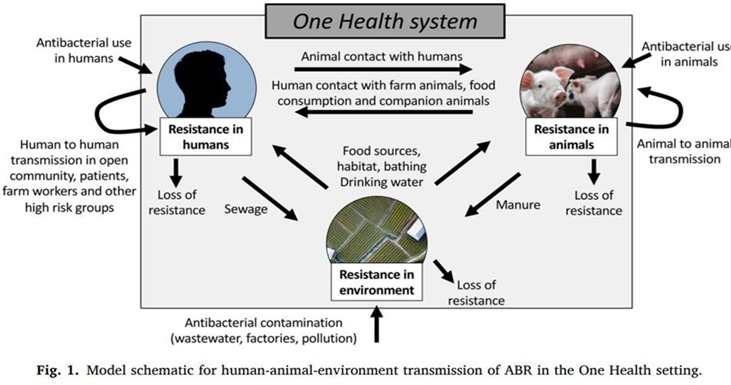
The One Health collaborative approach is aimed at improving communications and coordination between the partners in recognition of the interconnections between humans and animals. Figure 1 illustrates how, in the worst-case scenario, antibacterial resistance (ABR) can develop in either animals, environment, or humans and ultimately cross over and persist in a loop. However, there can also be some loss in resistance during crossovers, for example, a bacterium that develops resistance in the environment can lose the resistance when infecting animals.
These kinds of interconnections would be difficult to discover unless all sectors cooperate, share information, and collaborate effectively in developing strategies to reduce the spread and development of resistance.
The One Health approach by WHO aims to address microbial resistance by focusing efforts on:
- Improving awareness and understanding through education, training, and
- communication conduct surveillance and research to improve the knowledge and evidence base
- Encourage good Sanitation and Hygiene for the prevention of infections
- Optimise the Use of Antimicrobial Medicines in Human and Animal Health
- Develop an economic case for the even distribution of investments for all countries and increase investment towards new medicine, diagnostic tools, and vaccines.
Strategies for progress
Current strategies for controlling the use of antimicrobials are to identify drugs that are still effective for treating infections and categorised into groups reserved for human and veterinary medicines. On the 6th of July 2021, The Committee on the Environment, Public Health and Food Safety (ENVI Committee), a committee of the European Parliament, published a letter entitled “Drug resistance – criteria for identifying antimicrobial medicines reserved for treating humans”. The letter presents some positive results from bans and calls for the members to vote to remove more antimicrobials from the veterinary-approved list. The letter says Denmark no longer uses fluoroquinolones and cephalosporins of the third and fourth generation in animal farming and that there is no evidence suggesting animal health in Denmark has deteriorated as a result (European Parliament 2021).
Categories of use for antimicrobials as published by WHO;
- Category 1 – Antimicrobials used in veterinary medicine with low public health concerns
- Category 2 - Antimicrobials used in veterinary public health are estimated high
- Category 3 -Not approved for use in veterinary medicine – Human only
Further to the categories, risk management levels and the actions of use for the antibiotic for animals (Agency 2019).
- Avoid – Only to be used for humans.
- Restricted – Can only be used to treat existing clinical infection with restriction where alternatives are absent.
- Caution –Used in the lack of veterinary alternatives but abundant alternatives for human health
- Prudence – Can be used, considered low risk for human health. Prolonged and misuse is discouraged.
Conclusion
Research that examines the optimal use of antimicrobials (dose, interval, duration, exposure) in animal production environments is urgently needed to increase our understanding of how bacterial antimicrobial resistance develops, disseminates, and persists.
The challenge to reducing, carefully using or not using antibiotics to curb the development of multiple resistant microbes has been slow in history, and global adoption of restrictive policies continues to be slow today. However, the prospects are looking positive. All efforts aim to ultimately reach a point where antibiotics are only for therapeutic use, highly restricted for prophylactic applications, and none is used for growth promotion and still meeting the global demand for meat and animal products for human consumption.
For more info on references for the article or any questions, please contact your nearest technical advisor.
